Measuring Specific Latent Heat of Vaporization of Water
|
|
Objective
|
To enable the student to measure the specific latent heat of vaporization of water by an electric method |
|
Theory
|
The specific latent heat of vaporization is the amount of heat that is needed change 1 Kg of a substance in the liquid state into the gas state at the boiling point.
When an electric heater is immersed in a water, the electrical energy flowing in heater will be totally converted into heat energy in the water. From electricity, the electrical energy is given by E=Pt where "P" is the heater power in watts, "t" time in seconds and "E" electrical energy in joules. From heat, we know
that the heat gained by the water is given by H=mc(T2-T1) where "H" is heat energy in joules, "m" water mass in "Kg", "c" specific heat in "J/KgC", T1 water initial temperature in degrees Celsius before heating, T2 is the water temperature after heating in degree Celsius
Assuming there no heat lost to the surroundings of the water, we can say the heat energy gained by the water is equal to the electrical energy.
Heat energy gained by water=Electrical energy given by heater that is Pt=mLv. By solving for "Lv" we obtain Lv=pt/m. Thus from this equation we need the following data to measure the specific heat of the water
- Electric heater power. We can obtain this data from the specification of an electric heater
- Heating time: We need a stop-watch to measure the heating time
- Water mass: we need a balance to measure water that tuned into liquid
|
| Tools |
- Container: to hold the water
- Water: to measure its specific latent heat of fusion
- Lagging: to prevent heat from being lost to the surroundings
|
| Equipment |
- Electric heater : to supply heat
- Stop-watch: to measure time interval during which heat is supplied to water
- Balance: measure the mass of the water that melted

|
| Precautions |
- When heating the water, immerse the heater completely in the water
- Use lagging (heat insulators) to reduce heat loss to surroundings
|
|
Procedure and DATA
|
Method-I:
- Prepare all the needed tools and equipment
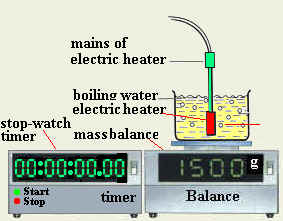
- Assemble the experiment as shown
- Place a quantity of water in the container
- Measure the mass of the water and container and record it as M1.
- Place the electric heater in water placed in container
- Switch on the heater
- Turn on the stop-watch when the water is boiling
- After several minutes, switch of the heater and the stop watch and record the mass of water as "M2"
- Record the time of the watch as "t"
- Find the mass of the evaporated water and record it as "m"
- Evaluate the latent heat of vaporization from Lv=pt/m
- repeat steps 7-11 and record three readings in the table
- Calculate the average of the three values obtained Lv
|
|
| |
Heater Power "P" |
|
Watts |
|
| |
Mass of water "M1" |
|
Kg |
|
| Try |
Stop-Watch time |
Mass of Container+Liquid |
Mass of evaporated water |
Calculated
Lv |
| |
t |
M2 |
m=M1-M2 |
Lv=pt/m |
| 1 |
|
|
|
|
| 2 |
|
|
|
|
| 3 |
|
|
|
|
| |
|
|
Average |
|
|
Method-II:
- Prepare all the needed tools and equipment
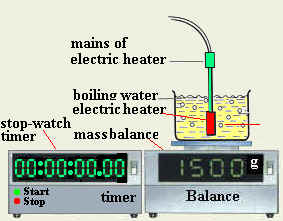
- Assemble the experiment as shown
- Place a quantity of water in the container
- Measure the mass of the water and container and record it as M1.
- Place the electric heater in water placed in container
- Switch on the heater
- Turn on the stop-watch when the water is boiling
- After each five minutes, record the mass of water and container as "M2"
- Record the time of the watch as "t"
- Find the mass of the evaporated water and record it as "m"
- repeat step "8-10" for five times
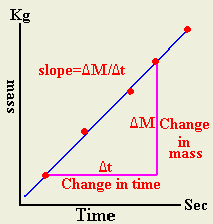
- Plot mass of evaporated versus time
- Evaluate the slop from the graph
- Calculate the heat of vaporization from m=(P/ Lv) t where slop=P/ Lv
-
|
 |
| Heater Power (P) |
|
|
| Mass of Water and container (M1) |
|
|
| Try |
Time |
Mass of Container+Liquid |
Evaporated Mass |
 |
| |
t |
M2 |
m=M1-M2 |
| 1 |
|
|
|
| 2 |
|
|
|
| 3 |
|
|
|
| 4 |
|
|
|
| 5 |
|
|
|
| |
|
|
|
|
|
| Analysis |
Questions
- What is the percentage error of your experiment? use %Error=(Actual-Experimental)/Actual
- If your experimental value is less than the actual value, then explain why?
- If your experimental value is greater than the actual value, then explain why?
- Name the objects that will gain heat other than the water?
- Why did we use the electric heater rather than using Bunzen flame?
- Why does the heater should be completely immersed in the water?
- Why should we the use the lagging?
- Why did we turn on the stop watch when water started to boil?
|