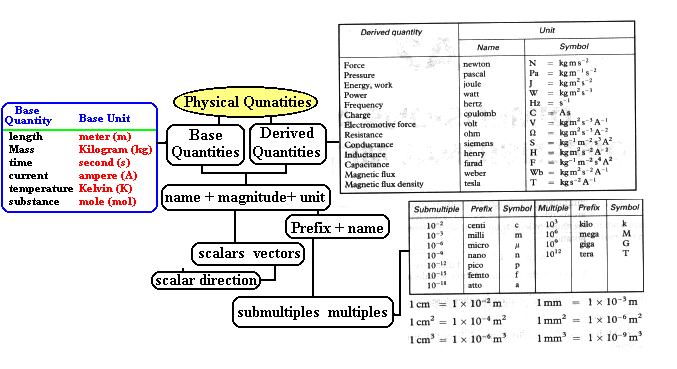
 | Quantities, Units, SI units |
 | Base Quantities, Base units |
 | Derived quantities, Derived units |
 | Homogenous, Correct/incorrect equations |
 | Prefixes |
Physical Quantities
- What is meant by a physical quantity?
A physical quantity is a measure to a physical
parameter.
- What are the two major components of physical
quantity?
1- Magnitude 2- Unit
- What does "SI" means?
It means International System of Units.
- What are the Base ( Basic) Quantities?
length, mass, time, current, temperature,
amount of substance
- What are the basic units in SI system?
Kilogram (Kg), meter (m), second (s), ampere
(A), Kelvin (K), mole (mol)
candela (cd)
- List the main definition of the Base units.
| Unit |
Abbv. |
Definition |
| metre (meter) |
m |
The metre is 1 650 763.73 of the wavelength, in vacuo
of the orange light emitted by 86Kr in the transition 2p10
to 5d5. |
| kilogram |
kg |
The kilogram is defined as the mass of a
platinum-iridium cylinder kept at Sevres, near Paris. This
definition is not recommended for work of high precision. |
| second |
s |
The time taken by 9 192 631 770 cycles of radiation
from the hyperfine transition in caesium when unperturbed by
external fields. |
| ampere |
A |
That constant current which, if maintained in each of
two infinitely long straight parallel wires of negligible
cross-section placed 1 metre apart in a vacuum, will produce between
the wires a force of 2 x 10-7 newtons per metre length. |
| mole |
mol |
The amount of substance which contains as many
elementary entities as there are atoms in 0.012 kilogram of the
carbon isotope 12C. |
| kelvin |
K |
The kelvin is the fraction 1/273.16 of the
thermodynamic temperature of the triple point of water. |
| candela |
cd |
The luminous intensity, in the perpendicular
direction, of a surface of 1/600 000 square metre of a full radiator
at the temperature of freezing platinum under a pressure of 101 325
newtons per square metre. |
What is meant by a derived unit, give an example?
Any unit not listed in based unit is called a
derived unit. A derived unit is usually formed by multiplying or/and
dividing the Basic-Units. Example: Force=mass*acceleration, Newton=kilogram* m/sec2.
The following table lists derived quantities and units. The table also
shows the formula from which the unit was derived.
| No |
Derived Quantity |
Formula |
Unit Name |
Unit Symbol |
| 1 |
Force |
F=ma |
newton |
N=Kg m s-1 |
| 2 |
Pressure |
p=F/A |
pascal |
Pa=Kg m-1s-1 |
| 3 |
Energy, work |
W=Fd |
joule |
J=Kg m2s-2 |
| 4 |
Power |
P=dw/dt |
watt |
W=Kg m2s-3 |
| 5 |
Frequency |
--------- |
Hertz |
Hz= s-1 |
| 6 |
Charge |
q=It |
Columb |
C=As |
| 7 |
Electromotive force |
P=VI |
volts |
V=Kg m2s-3A-1 |
| 8 |
Resistance |
V=IR |
Ohm |
W=Kg m2s-3A-2 |
| 9 |
Conductance |
G=1/R |
siemens |
S=Kg-1 m-2s3A2 |
| 10 |
Inductance |
e=Ldi/dt |
henry |
H=Kg m2s-2A-2 |
| 11 |
Capacitance |
C=Q/V |
farad |
F=Kg-1 m-2s4A2 |
| 12 |
Magnetic flux |
F=nEt |
weber |
wb=Kg m2s-2A-1 |
| 13 |
Magnetic flux density |
B=F/A |
tesla |
T=Kg s-2A-1 |
How do we simplify complex derived units. give
examples?
By naming them. Ohm=Kg m2 s-3
A-2
What is meant by a homogenous equation? Give
an example?
A homogenous equation is an equation where the
units of its right side are equal to the units of that of its left
side.
F=m a, [N]=[m][a], kg m s-2=kg m s-2. All
units on the two sides are equal
When do we refer to an equation as a correct
equation? Give example.
When both sides of an equation give the same
units, same numerical values, and same concept we refer to the equation as
being correct. The area of a sphere is A=4pr3.
This equation is correct because its sides give the same results.
How do we give a homogenous equation but an
incorrect equation at the same time?
- Removing constants from correct equations make
them homogeneous but incorrect. For example, the correct formula
for kinetic energy is K.E.=(1/2) m v2. If we remove the
constant 1/2 we get K.E=m v2, which is homogenous but
incorrect.
- Adding constants to correct equations make them
homogeneous but incorrect. For example, the correct formula for
pressure p=F/A. If we add a constant say "2" we get
p=2F/A, which is homogenous but incorrect.
- Mixing concepts with similar units. For example,
work, Kinetic energy, and potential energy have the same units but
saying the potential energy is P.E=(1/2)mv2 is incorrect.
- Mixing equations with similar terms. For
example, work is defined as W=F*D and torque is defined as G=F^*D.
So, the equation w=F^*D
is homogenous but incorrect.
What is meant by a unit-prefix? Give an example?
A prefix is used with unit symbols to indicate decimal
multiples or submultiples.
kilogram (prifix=kilo and unit is gram)
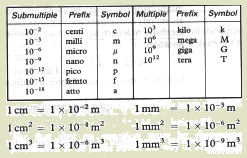 What are the common multiple-prefixes?
What are the common multiple-prefixes?
Kilo (k)=103, mega (M)=106, giga
(G)=109, tera (T)=1012
What are the common submultiples-prefixes? Give
examples
centi (c)=10-2, milli (m)=10-3,
micro (µ)=10-6, nano (n)=10-9, pico (p)=10-12, femto (f)=10-15,
atto (a)=10-18
Kilogram, nanosecond, 10 Mega bytes, 1 Giga Byte, 5 cm,
Test Problems
- With the aid of an example, explain the statement
"The magnitude of a physical quantity is written as the product of a
number and a unit"
- Explain why an equation must be homogeneous with
respect to the units if it is to be correct?
- Write down an equation which is homogeneous , but
still incorrect?
- Kepler discovered that the period T of the planets
about the Sun are related to their distances "r" from the
Sun. From Newton's laws, the following equations may be derived T2=(4p2/GM)r3.
Use the equation to find the units for the G in terms of base units.
- For each of the four concepts listed in the left
hand column, place a tick by the correct example of that concept in the
appropriate box.
| Concept |
Example |
Example |
Example |
| Base unit |
c
mole |
c
length |
c
Kilograms |
| Derived unit |
c
coulomb |
c
ampere |
c
volt |
| Base Quantity |
c
torque |
c
velocity |
c
kinetic energy |
| Derived Quantity |
c
mass |
c
density |
c
density |
Demonstrate that the following equation is
homogeneous with respect to units. The symbols have their usual
meanings. x=ut+(1/2)a t2.
Classify each of the units in the left-hand column
by placing a tick in the relevant box.
| |
Base unit |
Derived unit |
Base quantity |
Derived quantity |
| Length |
c |
c |
c |
c |
| Kilogram |
c |
c |
c |
c |
| Current |
c |
c |
c |
c |
| Power |
c |
c |
c |
c |
| Coulomb |
c |
c |
c |
c |
| Joule |
c |
c |
c |
c |
Each row in the following table starts with a term
in the left hand column. Indicate with a tick which of the three
expressions in the same raw relates to the first term.
| Joule |
kg.m.s-2
c |
kg.m2.s-2
c |
kg.m2.s-3
c |
| Coulomb |
Base
unit c |
derived
unit c |
Base
quantityc |
| Time |
Scalar
Quantity c |
Vector
Quantity c |
neither
vector nor scalarc |
| volt |
AxW
c |
AxW-1
c |
WxA-1
c |
Each row in the following table contains two correct statements and one
incorrect statement. Put a cross in each row against the incorrect
statement.
| c
Mass is base quantity |
c
Charge is a base unit |
c
Force is a derived quantity |
| c
Watt=joule/second |
c
Volt=joule/coulomb |
c
Coulomb=ampere/second |
| c
Gradient of displacement/time graph is velocity |
c
Gradient of velocity/time graph is acceleration |
c
Gradient of acceleration/time graph is displacement |
| c
mass is a scalar quantity |
c
linear momentum is a scalar quantity |
c
power is a scalar quantity |
Homework:
Questions 1A: 1,2,3,4
Questions 1B: 1,2,3
Concepts map
 |
